
( Paediatric Multi-inflammatory Syndrome temporally associated with SARS-CoV-2 )

Whether ultimately any close link is found between KD and PIMS, there is much that can be learnt from comparing them. KD has 60 years of research to draw on and PIMS-TS/MISC has the focus of the whole world and lots of scientific interest and funding at present. The differences are just as interesting as the similarities. Our greatest hope is that by collectively thinking out of the box and working together to join the dots, we can make breakthroughs in the understanding of both syndromes.
What is Kawasaki Disease?
Kawasaki Disease (KD) is an acute auto-inflammatory syndrome sometimes referred to as vasculitis, which generally affects children of <1 to <5 years, but can also affect older children and teenagers. The trigger for KD, a syndrome identified 60 years ago, is still unknown, but viruses including corona viruses have been cited as possible triggers. Genetics have been found to play a part, with more boys than girls, and certain ethnicities disproportionately affected. Symptoms include high unrelenting fever, rash, red eyes, cracked lips, swollen lymph nodes and red and swollen hands and feet. If diagnosis and treatment is delayed, there is a greater chance of permanent damage, which usually involves the arteries and the heart, and in severe cases, can result in giant aneurysms. Kawasaki Disease is the number one cause of acquired heart disease in children.
Temper’s symptom leaflet from Societi, the UK representative body for KD Tempers-A4_endorsed-Sept-2018.pdf
Gallery of KD symptoms with thanks to Leia’s family www.bachiw.co.uk/rashes
Looks and sounds familiar, doesn’t it?
In February 2020 when we first started to be aware of Covid-19, families with children who had Kawasaki disease (KD) started to notice similarities, particularly in those who became seriously ill with a cytokine storm, causing hyper inflammation and organ damage. Then in April, PIMS-TS arrived, and jaws dropped. To all intents and purposes, KD and PIMS-TS were the same. They both affected children, and both had the same symptoms. We knew what would be next. We would go on to find that they are more common in boys than girls and show signs of ethnic predisposition, just like KD. We knew that they would need very similar treatments, which of course they eventually did. Since then, a lot of discussion and research has taken place around the question: “are PIMS-TS and Kawasaki Disease the same?”
Differences and Similarities
- We do not know what the trigger for KD is, but we do know that COVID-19 is the trigger for PIMS-TS
- The median age of PIMS-TS children is generally higher than those with KD. However, although most common in younger children, KD is seen in older children too. In fact, very young and older KD children often present with incomplete or atypical symptoms. Also no one has had time to develop immunity to Covid, so this could explain why older children and some adults are getting PIMS.
- Both PIMS-TS and KD are more common in boys than girls in a ratio of 3:2
- PIMS-TS is more common in children of African, Afro-Caribbean and Hispanic decent and KD is more common in children of East Asian/Japanese and Pacific Islanders descent
- Symptoms are very similar. PIMS-TS does seem to have more abdominal pain, sometimes severe, which is less common in KD. PIMS-TS often shows toxic shock symptoms. (rapid heart rate, low blood pressure, unresponsive behaviour.) This does not happen with all KD cases but it does in some. PIMS-TS tends to present with myocardial disfunction, whereas KD tends to present with coronary aneurysms.
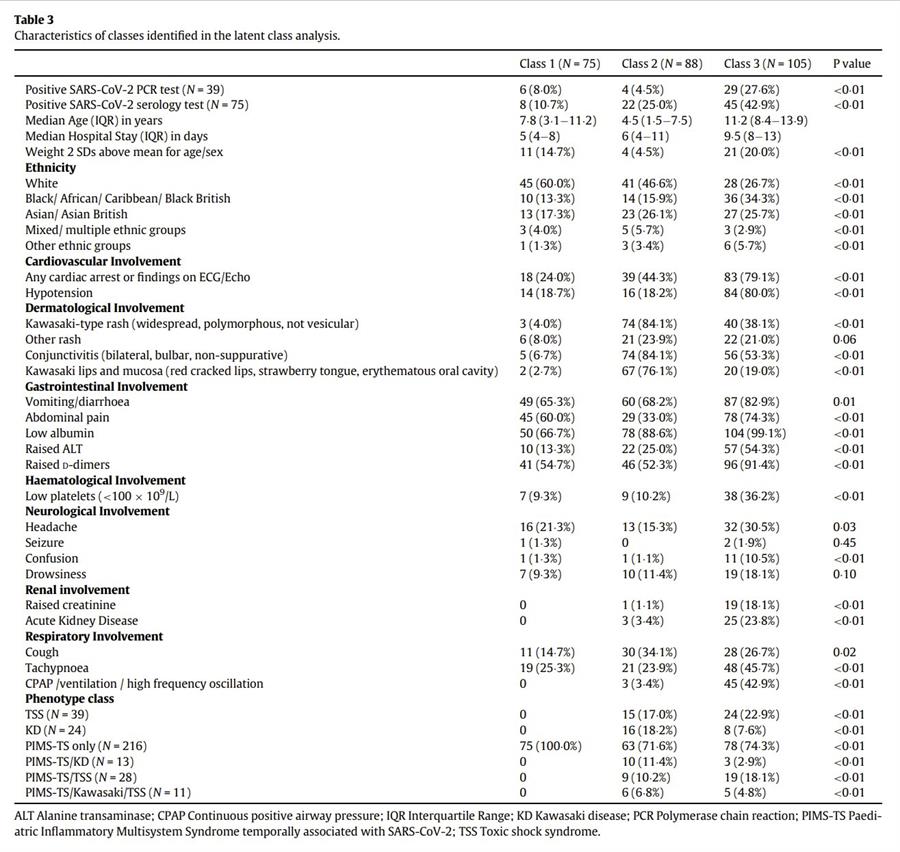
- PIMS-TS and KD do not always present in a textbook manner and if the distinction is unclear the diagnosis is sometimes given as PIMS/KD or Covid KD
- PIMS-TS is treated in virtually the same way as KD, with corticosteroids, IVIG and cytokine blockers such as Tocilizumab, Anakinra and Infliximab. Sometimes anticoagulants are used and low-dose aspirin as an antiplatelet therapy
- KD can also be atypical or incomplete, meaning that not all symptoms are present. These cases might not require hospitalisation and might go undiagnosed. They can still cause damage to the heart and arteries. PIMS-TS appears to be more acute and require hospitalisation, but the changes to the organs tend to resolve with treatment. It is not yet known if there are atypical or incomplete PIMS-TS cases and whether this will increase the number of children with undetected acquired heart disease.
- Some after-effects of PIMS-TS and KD are similar such as rash when unwell, headaches and joint pain, whereas mood swings and changes in behaviour, peeling and high temperatures when unwell seem to be more common in KD. Interestingly, some of the after- effects of Covid, for example Long Covid, are also very similar to PIMS-TS and similar to some of those in KD.

NIHR Imperial Biomedical Research Centre - Genetic signature diagnostic test for Kawasaki Disease
Other researchers such as Dr Rae Yeung are saying that even with some differences, the two conditions are on the same spectrum and that we should be looking at the bigger picture, allowing for crossovers and treating by presentation, not by name. (Source “What’s in a name” webinar)
Dr Kawasaki, who first discovered Kawasaki Disease, originally filed the condition as GOK, short for “God only knows”. Although much progress has been made in the 60 years since then, the cause or trigger is still unknown and there are still more questions than answers.
Some of the suggested triggers for KD over the years:
Adenovirus, herpesvirus, streptococci, Rickettsia species, Epstein Barr virus, retrovirus, human coronavirus- New Haven,
measles virus, house dust mite, mercury, carpet shampoo, residence near a body of water, and most recently SARS-COV-2.
Below is a synopsis of the most recent research on KD, more detail can be found on the KD Annual Symposiums.
2020 KD Symposium www.youtube.com/watch
2021 KD Symposium www.youtube.com/watch
In Japan, which has the highest case numbers globally, there have been three KD epidemics (April 1979, May 1982 and March 1986) Research has shown that these epidemics were linked to the change in wind direction in Japan at that time and that more generally, localised north westerly winds coincided with greater numbers of KD. So, could the global winds be carrying the infectious agent or trigger for KD? This interesting idea has been researched in Hawaii and San Diego, which also show high cases of KD compared to elsewhere globally. The wind pattern theory was compatible with spikes in cases of KD there too. This is a major shift in thinking, as long- range wind transportation of an infectious agent was not thought possible. The origin of the infectious agent, based on the direction of the wind, appears to be North-East China, an intensely agricultural area. Candida, found in aerosol samples of the wind when this research was being carried out, was considered a possibility. But questions still remain as to what the infectious agent might be, or whether in fact there is more than one trigger for KD. This area of research is primarily led by Dr Jane Burns and her team at Rady Children’s hospital, USA

This has also been investigated and Dr Anne Rowley has led the way in this area. She suggests that if there were multiple triggers for KD, epidemics and clusters would be less likely to be observed. She suggests the trigger to be an RNA virus that is inhaled and enters the body either by direct infection or by shedding from a previously infected person. This might create different viral loads that might then trigger differing presentations and severities of KD. The virus then enters the system though the bronchial epithelium. The infection itself is often asymptomatic, with KD then appearing in genetically susceptible children. Dr Anne Rowley has used synthetic antibodies to identify an antigen in respiratory epithelial cells from children with KD. Hepacivirus is one possible trigger that has emerged. (April 2019)
A Hepacivirus-Like Protein Is Targeted by the Antibody Response to Kawasaki Disease
www.frontiersin.org/articles.
Seasonal influences
KD has distinct seasonal peaks, which although in certain years have moved slightly, generally occur from Jan-March in the Northern Hemisphere and May-June in the Southern Hemisphere and tropics. This is thought to link to seasonal winds and colder, wetter weather.
The weather and environmental theory
Research by Professor David Burgner and others has also found that cases of KD are more common at times of low temperature and high rainfall and also more common in rural areas. Increased_KD_associated with Precipitation
But this raises the question of why? Do these conditions make the trigger more accessible in the outside environment? Or do we spend more time inside and encounter the trigger inside, or is it a complicated combination of the two?
And does this relate to why other viruses such as flu and the common cold are more common in the winter months? If it is linked to weather, localised weather conditions might perhaps explain the clusters of KD cases observed? Clusters of KD have often shown shared clinical presentations and similar measures of inflammation, perhaps indicating a specific local trigger?
Or perhaps that in the pandemic, in periods of lockdown, air pollution dropped dramatically, and the trigger was less in evidence?

Social and Behavioural influences
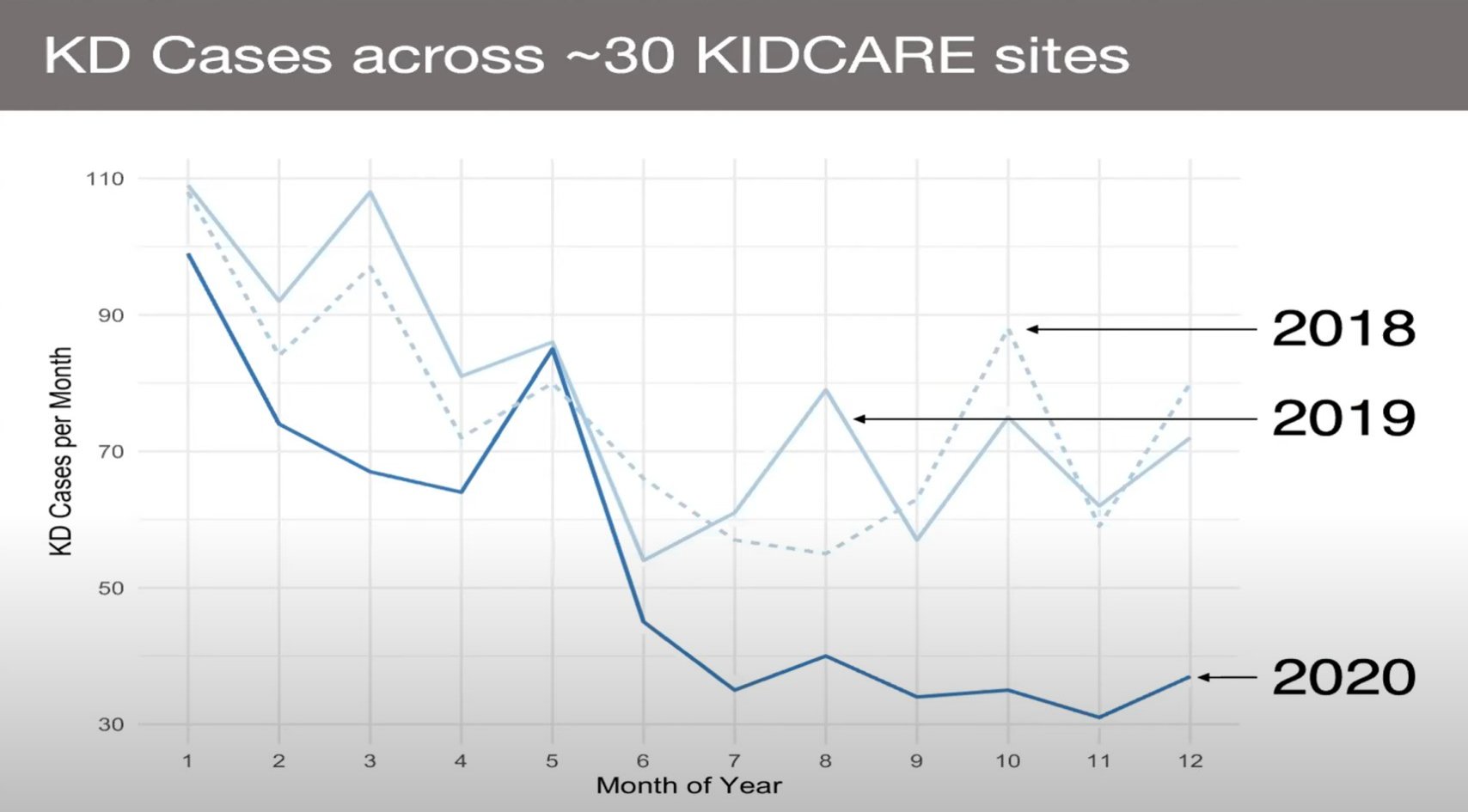
Research in the USA is showing that KD cases went down during the pandemic, particularly in lockdown. They did not disappear completely, however.
Unless one thinks that KD is PIMS-TS, with the same trigger, then we have to consider what has changed. It is unlikely that the trigger for KD has disappeared and indeed, KD cases are beginning to reappear after re reopening of society (USA research) So, is it a change of behaviour, for example, how we spent more time indoors in the lockdown, where person to person transmission (PIMS) is more likely than an environmental trigger (KD)? Did the reduction in air pollution play a part? Or is it that children were not at school and were not mixing or becoming infected by peers, in which case the KD trigger could be spread person to person?
Genetics
Children of Japanese ethnicity have always had a greater predisposition to KD, wherever they live in the world. Interestingly, cases of PIMS-TS/MISC have not been seen in Japan and Korea, suggesting that they are not genetically predisposed to PIMS-TS and therefore haven’t been triggered to get ill with it? Or is it that they are so familiar with KD in Japan, that they have diagnosed PIMS-TS as KD, as to them it is not something new?
Following on from the above, other interesting observations of KD have been made during the pandemic.
- The clinical presentation has changed slightly, with less children showing strawberry tongue, swollen lymph nodes or peeling hands and feet. Of all these, peeling hands and feet has not yet reappeared since cases of KD have begun to return to pre-pandemic levels in the USA*
- Less boys, less Asian and Hispanic children and less 1–5-year-olds were presenting with KD in the USA *
- More children from higher income brackets got KD in lockdown in the USA*
- Data in the UK is very sparce, but indications show that KD cases have not been much lower than usual during the pandemic, we also do not know if they are presenting any differently.
*Source KD symposium 2021

- KD cases have been steadily rising over the last 10 years, why is this?
- If the trigger for KD could be a coronavirus, then what do we know about the growing global prevalence of corona viruses over the last 10 years?
- PIMS-TS occurs 2-6 weeks after Covid infection. If KD could also be triggered by a coronavirus, perhaps we should be looking further back from onset of symptoms and using antibody tests as well as swab tests and testing for a wider range of viral triggers.
- Have children of East Asian/Japanese ethnicity living elsewhere in the world been affected by PIMS-TS?
- Have KD cases been lower in all parts of the world during the pandemic period and if so, why do we think this is? Does this tie in with lockdowns? Lower pollution rates? Other reasons?
- Is it possible to estimate what % of child covid cases have resulted in PIMS, ie what % of children have a genetic predisposition to PIMS? Does this tally with what we know about KD?
- If annual PIMS case numbers are no higher than annual KD numbers, in a pandemic, what does that say about the possible trigger/triggers for KD?
- Could it be that KD is the same as PIMS-TS, just triggered by a slightly different superantigen, perhaps even another corona virus that has been around for longer and produces slightly different symptoms and outcomes in slightly different aged children
- What will happen if and when Covid 19 abates. Will PIMS disappear and what will happen to KD?
- Now that most countries are no longer in lockdown, how has that affected KD numbers across the globe?
KD has presented many interesting clues over many years. To find answers that will hopefully shine a light on both KD and PIMS-TS, we must all keep thinking and asking questions.
If you have an out of the box question, please email it to us on info@PIMS-Hub.org.uk and we will add it in.
Societi
UK Facebook KD Support group
KD Facebook Awareness Group
KD Foundation
Further Reading
Viral infections associated with Kawasaki disease - ScienceDirect
Kawasaki disease recurrence in the COVID-19 era: a systematic review of the literature
Could Sars-Cov-2 trigger a recurrent case of KD?
KD complications in adulthood
ncbi.nlm.nih.gov/pmc/articles/PMC2870533







